History and Evolution of Breast Augmentation: A Journey to Natural Breast Enhancement
Discover the fascinating journey of breast augmentation through the ages, from bust creams to modern innovations like breast implants, BRAVA, and now EVEBRA.

1897
Sears Roebuck introduces the “Princess Bust Developer,” a toilet plunger-like pump device claiming to enlarge breasts. Unfortunately, it fades into obscurity like many remedies of the time.

1962
Renowned plastic surgeons, Dr. Tom Cronin and Dr. Tom Biggs—esteemed mentors of Dr. Roger Khouri—make history by performing the first-ever breast augmentation with silicone breast implants. This influential moment propelled the evolution of modern breast augmentation.
*The first silicone breast implant, envisioned by Dr. Thomas Cronin and Dr. Frank Gerow, put into a human patient, Timmie Jean Lindsey, in 1962. Credit: Courtesy of Dr. Thomas Biggs. Courtesy of Thomas Biggs

1977
The “Salonmay Bustline Increaser” enters the market as a novelty device, often found in mail orders and adult stores. Numerous copycats, including the likes of the Noogleberry, follow suit. However, these inexpensive gadgets lack the engineering required to sustain a safe vacuum level over the necessary duration for tissue growth. At best, they induce temporary edema swelling and skin bruising. These devices are considered gadgets and there is no scientific proof that they offer any lasting enlargement.

1987
Liposuction emerges as a revolutionary technique for fat grafting. Some plastic surgeons experiment with injecting the extracted fat into the breast for enlargement. Unfortunately, this initial approach faced challenges, as the transplanted fat did not survive, leading to complications. Consequently, the American Society of Plastic Surgeons imposed a ban on fat grafting to the breast. Implants remained as the only method of breast augmentation. Millions of women worldwide opt for breast augmentation with silicone implants.
1992
The FDA puts a moratorium on silicone implants due to concerns that implants may trigger health problems. The moratorium was later reversed, but the health-related ill effects of breast implants still linger today. Lymphomas, squamous cell carcinomas, capsular contractures, and an ill-defined breast implant illness continue to plague breast implants. The FDA continuously releases warnings about breast implants.

1996
By this point, Dr. Khouri has successfully completed over 1000 breast reconstructions using microvascular free flaps (DIEP). This intricate procedure involves reconstructing the missing breast by extracting tummy tuck tissue from the lower abdomen and transferring it to the chest to create a breast mound. The operation is highly delicate and complex, requiring microscopic connection of the arteries and veins to ensure the tissue's survival in its new location. Dr. Khouri, widely acknowledged as a leading expert in this field, recognizes the challenges with the extensive, invasive nature of the surgery. It clearly involved significant cutting and carried a notable risk of complications. Driven by the pursuit of a better alternative, he kept innovating.
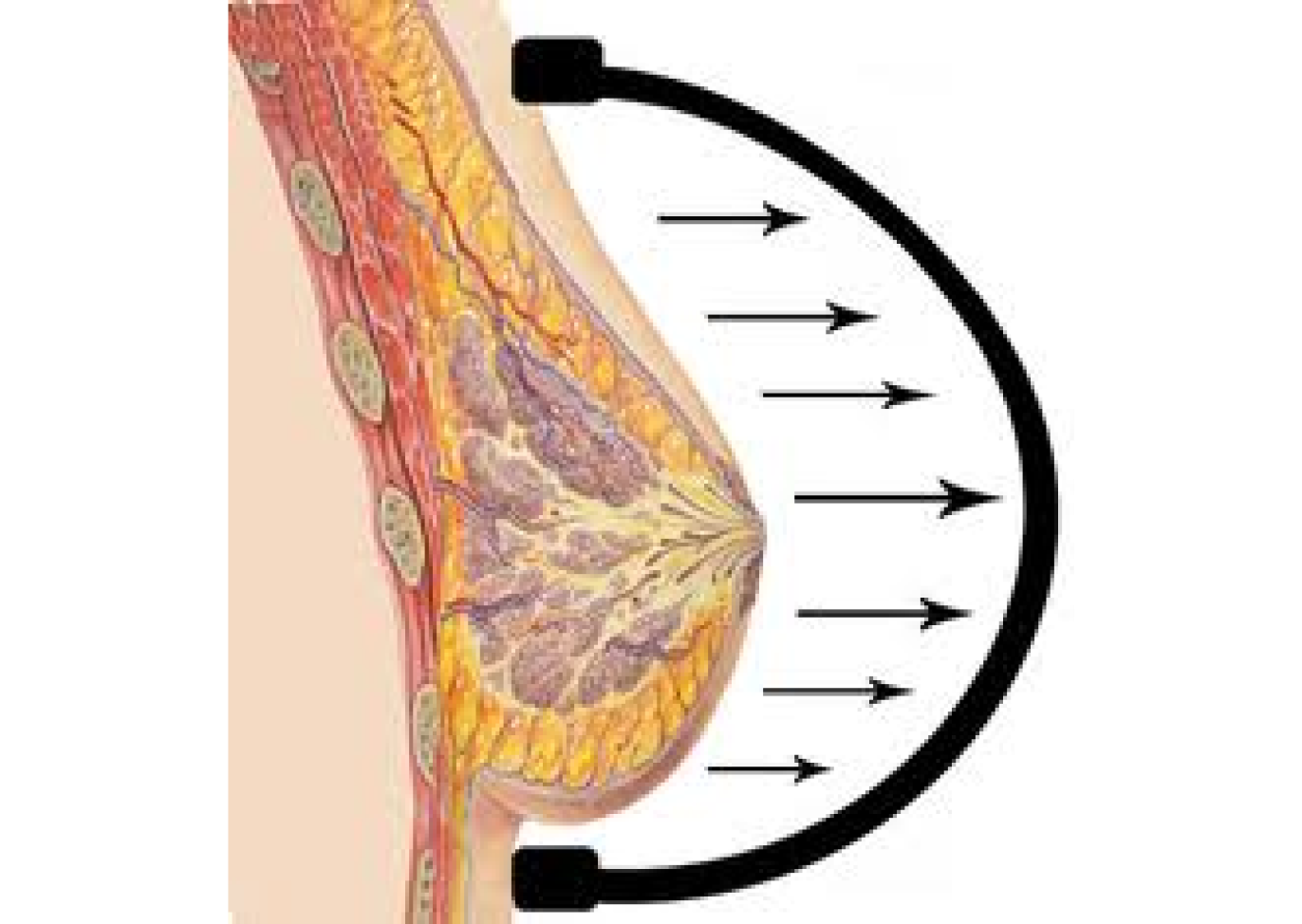
1998
Drawing inspiration from the common use of tissue expanders in orthopedic and plastic surgery, and grounded in the well-established science of mechano-transduction (tissue growth in response to stretch), Dr. Khouri hypothesizes that external tissue expansion could effectively enlarge the breast. However, to induce tissue growth, this external expansion must be consistently and applied in a controlled fashion over an extended period time. Something the novelty breast suction device gadgets and gimmicks on the market could not deliver. Collaborating with biomedical engineers, Dr. Khouri designed, prototyped and tested a meticulously engineered external breast expansion device. This device was capable of being worn as a bra over several weeks.

1999
Dr. Khouri conducts a well-controlled clinical study to demonstrate the efficacy of his newly developed external breast expansion device. Women who adhered to the treatment protocol experienced a 55% enlargement of their breasts during long-term follow-up. Dr. Tom Baker, his co-investigator and a prominent figure in the world of plastic surgery was awarded best research of the year when he presented these ground breaking results at the Annual Meeting of the American Society of Aesthetic Plastic Surgery. This scientific study was published in the prestigious Journal of Plastic and Reconstructive Surgery.

2001
The FDA completes its review of the scientific data behind this new device and grants its approval to be marketed for breast enlargement. BRAVA, a revolutionary device, is introduced to the market, marking the debut of the first scientifically proven non-surgical device for breast enlargement. Numerous subsequent publications validate the safety and long-term effectiveness of breast augmentation with Brava. See scientific references.
2005
Success stories of Brava are highlighted in prestigious publications such as ELLE, ALLURE, Newsweek, LA Times, and others. Additionally, a noteworthy epidemiologic study conducted by Dr. Gary Brody, the plastic surgeon credited with uncovering the link between textured implants and breast lymphoma, revealed that women who utilized Brava experienced a reduced incidence of breast cancer. This groundbreaking discovery led to the filing of a US patent, recognizing this technology as a potentially cancer-protective device.

2006
Over 60,000 women worldwide have experienced the benefits of Brava, clearly demonstrating its effectiveness in breast enlargement with enduring results. While the scientific foundation was established, challenges with compliance persisted. Brava, though effective, posed difficulties in wearability. Initial swelling and temporary enlargement were evident, but true tissue growth was a slow process demanding commitment, time and discipline. Brava primarily suited patients with patience and didn't replace breast implants for augmentation.
Recognizing the temporary enlargement achieved by Brava as an optimal foundation for fat grafting, Dr. Khouri secured ethics committee approval to revisit a previously banned operation. Collaborating with renowned plastic surgeons, he conducted clinical studies affirming the safety and efficacy of Brava's short-term use in preparing the breast for reconstruction and augmentation through fat grafting.
2008-2015
Dr. Khouri shares the outcomes of his comprehensive clinical studies on successful fat grafting to the breast with Brava pre-expansion. His noteworthy contributions earn him the Best Presentation award at the esteemed European Association of Plastic Surgeons (EURAPS) and recognition for the Best Papers published in the Journal of Plastic and Reconstructive Surgery.
2009

The data presented by Dr. Khouri, along with insights from other pioneers such as Emmanuel Delay in France, Gino Rigotti in Italy, Kotaro Yoshimura in Japan, and Scott Spear and Syd Coleman in the USA, played a pivotal role in persuading the American Society of Plastic Surgeons to lift the twenty-two-year ban on fat grafting to the breast. The Society acknowledges that, when executed properly, fat grafting to the breast is effective and entails minimal complications.
2015
Brava is now often used as an off label device to prepare the breast for fat grafting. When the Brava company asked the FDA to approve the very same Brava device authorized for sale directly to consumers for breast enlargement to now change the indication of use to prepare the breast for fat grafting, the FDA reclassified Brava as a Class III device if it is used for that new indication. (Class III are the medical devices that represent a high risk to the patient. These devices usually sustain or support life, are implanted, or present potential unreasonable risk of illness or injury). As a result of this ruling, the FDA prohibits the company from selling Brava for fat grafting unless it gathers the extensive clinical research data required to obtain Class III approval.
2016

2017

2023

Dutch plastic surgeons conducted a prospective randomized study, the highest level of scientific validity, to compare breast reconstruction with EVE + fat grafting versus tissue expansion + implants. The results, published in the prestigious JAMA magazine, overwhelmingly favored EVE + fat grafting. Women not only reported higher satisfaction with reconstruction using EVE + fat grafting, but there were also fewer complications, leading to reduced healthcare costs. Subsequently, the Dutch Government Health authority approved EVE + fat grafting as a covered and preferred method of post-mastectomy breast reconstruction. https://jamanetwork.com/journals/jamasurgery/article-abstract/2802106
https://www.jprasurg.com/article/S1748-6815(23)00327-3/fulltext
2023
Convinced that tissue expansion alone can effectively enlarge the breast, Dr. Khouri sought to create a device more comfortable and practical than Brava/EVE. Collaborating with top engineers and utilizing modern materials, he developed EVEBRA. While still fundamentally an External Vacuum Expander (EVE), EVEBRA distinguishes itself significantly from Brava.
Over the past two years, Dr. Khouri has provided EVEBRA to his patients, and those who have used both devices can attest to a night-and-day difference. EVEBRA is discreet, causing no skin irritation, and wearability is no longer a significant burden. Patients can comfortably wear it almost all the time and achieve excellent results.
Differences between Brava/EVE and EVEBRA are notable:
10 minutes of BRAVA/EVE and EVEBRA USE

EVEBRA addresses the potential skin irritation of Brava/EVE by replacing the adhesive with a soothing lubricating cream. This allows the device's contact surface to maintain the vacuum seal by gliding over the skin and wrapping around the torso without irritation, efficiently dissipating damaging shear forces and counter pressure.
Additionally, EVEBRA features a series of interchangeable domes. Users initiate their breast enlargement journey with a dome barely larger than their breast, contributing minimally to their normal bust size. As the breast gradually enlarges over time with consistent use, users progress to the next larger dome. This pattern continues, ensuring that EVEBRA is never much larger than the user's usual bra size.



